Conversion of an alcohol to an alkyl halide:
However, the reaction of primary and secondary alcohols with hydrogen halides can frequently be a problem as unwanted rearrangement reactions often occur.
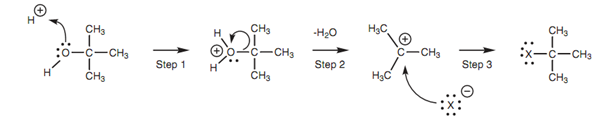
Figure: Conversion of alcohols to alkyl halides.
To prevent this, alternative procedures performed under milder basic conditions have been employed with reagents like thionyl chloride or phosphorus tribromide as shown in figure. These reagents work as electrophiles and react with the alcoholic oxygen to make an intermediate in which the OH moiety has been transformed into a better leaving group. A halide ion is released from the reagent in this procedure, and this can act like the nucleophile in the subsequent SN2 reaction.
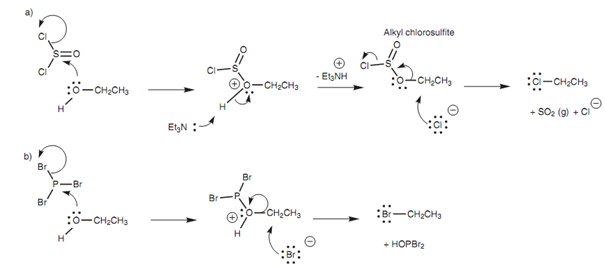
Figure: Conversion of an alcohol to an alkyl halide using (a) thionyl chloride; (b) phosphorus tribromide.
In the reaction along with thionyl chloride, triethylamine is available to mop up the HCl made during the reaction. The reaction is as well helped by the fact that one of the products (SO2) is lost as a gas, so driving the reaction to completion.
Phosphorus tribromide comprises three bromine atoms present and each PBr3 molecule can react with three alcohol molecules to make three molecules of alkyl bromide.