Phenomenon:
Now, let us first understand this phenomenon qualitatively. You know that all substances are made of molecules and the molecules interact with each other. The intermolecular force which enables same types of molecules of a given substance to attract each other is called cohesive force and this process is called cohesion. In solids, the cohesive forces are very strong. On the other hand, cohesive forces are weak for liquids and weakest for gases. That is why solids have definite shape, liquids have definite free surface and gases have neither. The force of attraction or repulsion between unlike molecules is called adhesive force and the process is called adhesion. Adhesive forces come into play at the common surface of two different substances. For instance, solder adheres to brass, glue adheres to wood, water adheres to glass etc. On the basis of these concepts, you can understand the surface tension related characteristics of liquids mentioned above. Let us discuss some of them now.
Due to the cohesive force, molecules at the surface of a liquid attract each other more strongly than the molecules in the interior of the liquid (Figure 1). It is so because the surface molecules do not have neighbouring molecules above the surface and, therefore, there are lesser number of molecules to share the cohesive force. This enhancement of cohesive forces among the molecules at the surface gives rise to a well-defined surface to liquids and the liquid surface behaves like a stretched membrane (something similar to a rubber sheet) tending to have minimum surface area. You might ask: How does this explain spherical shape of a liquid drop? Well, there is a small geometry include here. You may recall that for a given volume, the surface area of a sphere is minimum. Therefore, a drop of liquid must attain a spherical shape to have minimum surface area.
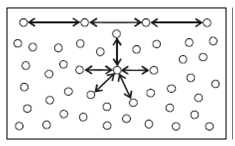
Figure: Two-dimensional View of the Molecules