Oxides and oxoacids
The most important oxides of all three elements (E) are EO2 and EO3. Sulfur additionally forms several oxides of low thermodynamic stability, for instance S8O with a structure consisting of an S8 ring. Sulfur dioxide SO2 is the main result of burning organic sulfur and sulfur compounds in air and is a severe air pollutant giving rise (after oxidation to H2SO4;) to acid rain. SO2 is a bent molecule and comprise both Lewis acid and basic properties with one lone-pair. For Reactions with strong oxidizing agents the liquid is a good solvent. SO2 dissolves in water giving acid solutions consisting of the pyramidal hydrogensulfite (HSO3-) and sulfite (SO32-) ions. The supposed sulfurous acid H2SO3, though, is exist only in very low concentrations. SeO2 and TeO2 comprise polymeric structures and give oxoacid salts identical to those from sulfur.
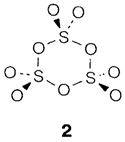
Sulfur trioxide SO3 is prepared industrially as a route to sulfuric acid, through oxidizing SO2 with oxygen by using a vanadium oxide catalyst. It can be present like a monomeric planar molecule but readily provides cyclic S3O9 trimers and linear polymers with corner-sharing SO4 units. The extremely exothermic reaction with water gives sulfuric acid H2SO4, that is the world's main industrial chemical, being used in several large-scale processes for creating soaps, fertilizers, dyestuffs, and detergents, and synthetic fibers. Anhydrous sulfuric acid goes through a series of acidbase equilibria like
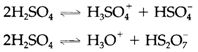
It is a extremely strong acid medium, where HNO3 (a strong acid in water) acts as a base:
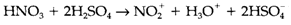
The resultant 'nitrating mixture' is employed for preparing aromatic nitro compounds through electrophilic reactions of NO2+ .
Reaction of HF with SO3 provides fluorosulfonic acid HSO3F that is than sulfuric acid more strongly acidic. In mixtures with SO3 and powerful fluoride acceptors like SbF5 it provides superacid media, that are able of protonating even several organic compounds.
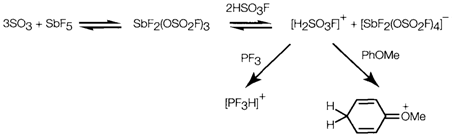
Diagram 2. Reactions in 'superacid' solutions.
SeO3 and selenic acid H2SeO4 are identical to the sulfur analogs apart from they are more strongly oxidizing. Tellurium behaves in a different way, as telluric acid has the octahedral Te(OH)6 structure, that, as supposed from Pauling's rules, is a extremely weak acid.
There are several other oxoacids of sulfur, of which the most significant are peroxodisulfate S2O82-that comprise a peroxo (O-O) bond, and compounds with S-S bonds include thiosulfate S2O32-dithionite S2O42-and tetrathionate S2O62- . The reaction
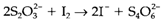
is employed for the quantitative estimation of I2 in aqueous solution.