Halides
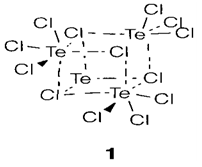
A selection of the most significant halides is illustrate in Table 1 and routes to the preparation of sulfur compounds are depicted in diagram 1. With sulfur the fluorides are extremely numerous and stable, but Se and Te depict an increasing variety of heavier halides. Compounds like S2Cl2 and S2F10 have S-S bonds; S2F2 has other isomer S=SF2. Sulfur halides are molecular and monomeric with structures supposed from VSEPR (example SF4 'see-saw', SF6 octahedral;). Increasing polymerization is found with the heavier elements, like in (TeCl4)4 (1) and related tetramers.
Table 1. Principal halides of S, Se and Te
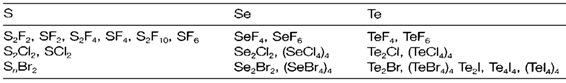
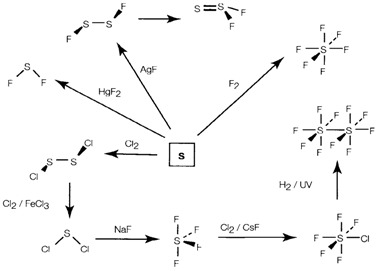
Fig. 1. Routes to the preparation of sulfur halides.
The hexahalides are kinetically inert but several other halides are extremely reactive and are hydrolyzed in water providing oxoacids and oxides. Intermediate hydrolysis results are oxohalides of which sulfuryl chloride SO2Cl2 and thionyl chloride SOCl2 are industrially significant compounds.
Some of the halides depict donor and/or acceptor properties. For instance, SF4 reacts with both Lewis acids (creating compounds like [SF3]+[BF4]-) and bases (forming either simple adducts like C5H5N:SF4 with pyridine, or compounds consisting of the square pyramidal ion [SF5]-). The complex ions [TeX6]2- and [SeX6]2 (X=Cl, Br, I) are interesting like they appear to have general octahedral structures despite of the existence of a nonbonding electron pair on the central atom.