Mechanism for the nitration of benzene:
The mechanism for the nitration of benzene is extremely identical to sulfonation as shown in figure.
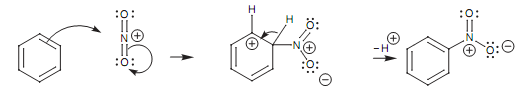
Figure: Nitration of benzene.
As the aromatic ring create a bond to the electrophilic nitrogen atom, a π bond among the N and O breaks and both electrons move onto the oxygen atom. Not like sulfonation, this oxygen keeps the negative charge of it and does not pick up a proton. This is since it works as a counterion to the neighboring positive charge on nitrogen.