Nomenclature:
Fatty acids are named according to the total number of carbon atoms and to the number and position of any twice bonds. The systematic names for fatty acids are developed through adding 'oic acid' on to the name of the parent hydrocarbon. Moreover, as fatty acids are ionized at physiological pH they are commonly written as RCOO- and have names ending in 'ate' rather than 'oic acid'. A C18 saturated fatty acid would be known as octadecanoate and a C18 monounsaturated fatty acid octadecenoate, and a C18 fatty acid with two twice bonds octadecadienoate. Yet, several nonsystematic names are still in use.
There is also a shorthand notation to show the number of carbon atoms and the number of any double bonds in the structure. With 18 carbons a fatty acid
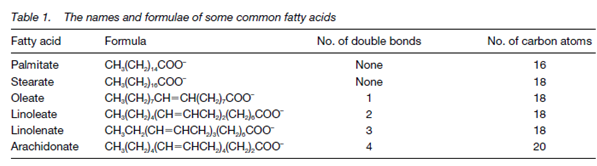
and no double bonds is designated 18:0, although one with 18 carbons and two double bonds is 18:2. An carbon atoms in fatty acids are numbered from the carboxylic acid residue and so the position of double bonds can be defined using the number of the first carbon included in the bond for instance Δ9 shows a double bond among carbons 9 and 10 of the fatty acid chain that is also described in the figure. The configuration of the double bonds in most unsaturated fatty acids is cis; so called because the two hydrogens on the carbon atoms either side of the double bond are on the similar side of the molecule Latin, cis = on this side of. Therefore, the full systematic name of linoleate (Table 1) is cis, cis-Δ9, and Δ12-octadecadienoate. In during the degradation of fatty acids some trans-isomers are establish, where the hydrogens on the carbon atoms either side of the double bond are on opposite sides of the molecule (Latin, trans= across).