Mass Spectrum:
i) The m/z value of the molecular ion, M gives the molecular mass and can also be used for generating the molecular formula.
ii) The relative intensities of [M + 1] and [M + 2] peaks can be related to the number and nature of hetero atoms present in a molecule. A typical pattern of M+1 and M+2 peaks is observed if a chlorine or bromine atom is present in the molecule.
iii) The odd molecular mass is indicative of the presence of a nitrogen atom in the molecule. Therefore, this has to be further confirmed through other means or by analysing the fragmentation patterns for the typical nitrogen containing functional groups.
iv) The characteristic peaks arising from typical fragmentation patterns of various classes of functional groups such as α cleavage, loss of little molecules like as H2O, C2H4, etc. are quite useful.
v) speaks which may be attributed to the rearrangement of the molecular ion or its fragments ions also give significant structural leads.
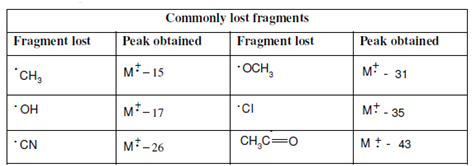
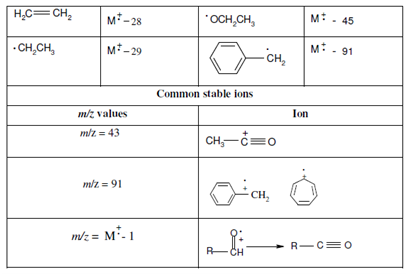
Table containing commonly lost fragments and stable fragment ions observed in the mass spectrum is being reproduced here so as to facilitate you in the interpretation of the mass spectra of the examples being taken up in the next section.
Table: Some commonly lost fragments and the stable fragments in the mass spectrum