Reactions of propanone and propanal with HCN:
This influence will be more important for a ketone in which there are alkyl groups on either side of the carbonyl group. An aldehyde has just only one alkyl group attached and thus the carbonyl group is much more accessible to nucleophilic attack.
We shall now come across at how steric factors that influence the stability of the transition state leading to the last product. For this reason we shall look at the reactions of propanone and propanal with HCN to provide cyanohydrin products.
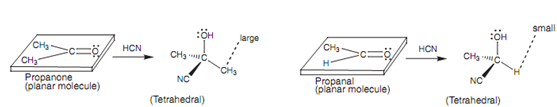
Figure: Reactions of propanone and propanal with HCN.
Both of the propanone and propanal are planar molecules. The cyanohydrin products are tetrahedral. So, the reaction leads to a marked variation in shape among the starting carbonyl compound and the cyanohydrin product. There is as well a noticeable difference in the space available to the substituents related to the reaction site - the carbonyl carbon. The tetrahedral molecule is more crowded because there are 4 substituents crowded round a central carbon, while in the planar starting material, there are just only three substituents attached to the carbonyl carbon. The crowding in the tetrahedral product taking place from the ketone will be greater as compared to that arising from the aldehyde because one of the substituents from the aldehyde is a small hydrogen atom.