Propanone:
The easiness with which nucleophilic addition occurs depends upon the ease with which the transition state is created. In nucleophilic addition, the transition state is thought to resemble the tetrahedral product much more than it does the planar starting material. Hence, any factor that affects the stability of the product will as well affect the stability of the transition state. Because crowding is a destabilizing effect, the reaction of propanone should be harder than the reaction of propanal. Hence, ketones generally will be less reactive as compared aldehydes.
As bigger the alkyl groups, the bigger the steric effect. For inctance, 3-pentanone is less reactive as compared to the propanone and fails to react with the weak bisulfite nucleophile while propanone does.
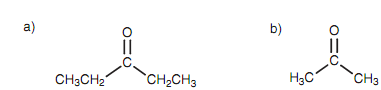
Figure: (a) 3-Pentanone; (b) Propanone.