Iron-Carbon System Phase Diagram
Carbon and Iron make a series of alloys that comprise a number of cast iron and steels. Steels are the alloys that comprise upto 1.2 percent of carbon whilst cast irons contain carbon inside range of 2.3 percent to 4.2 percent. An alloy along with carbon greater than 4.6 percent has poor properties and thus not utilized.
The carbon atom is minor than the iron atom or the diameters being 1.54 Å and 2.56Å respectively and in iron suspend interstitially. Pure molten iron solidifies at 1539oC in bcc structure. On 1400oC on additionally cooling such structure change into fcc and again at 910oC back to bcc. These three structures in reverse order that is form room temperature are named as a, g and d.
The a-iron is ferromagnetic although loses its ferromagnetism while heated above 770oC curic temperature. The non-magnetic a-iron was earlier called as b-iron until X-ray studies showed such structures of a - and d - irons are similar. The g-iron is the structure of closest packing whereas a - and b - irons are not. There will be abrupt modifies of dimensions at transitions a to g and g - b. Following figure depicts the cooling curve of pure iron.
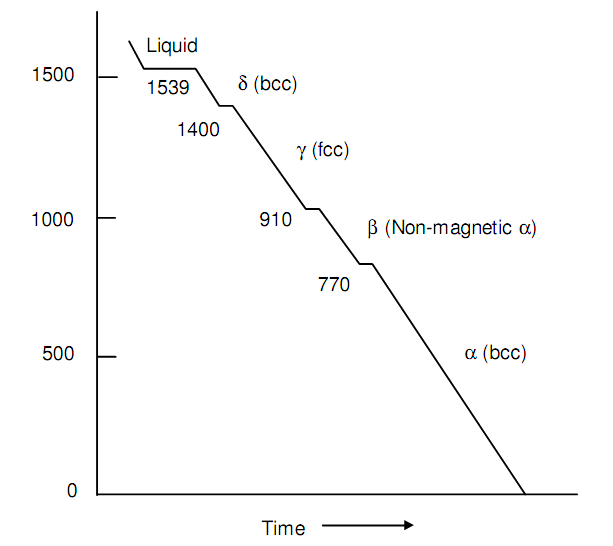
Figure: Cooling Curve of Pure Iron with Steady Rate of Heat Loss
Alloys in iron-carbon schemes also undergo complex structural changes that play an imperative role in deciding their characteristics. Modifies that arise in iron-carbon system are demonstrated in iron-carbon phase diagram of following figure. Strictly speaking the diagram concerns to the iron-iron carbide system although still the phase relationship can be expressed in terms of carbon percentage, thus the name.
Iron-carbon diagram is splits in different phase fields characterized by the existence of one or mix of two phases. The liquids above that merely molten metal in liquid state can exist are well-known as ABCD. Dissolved carbon and iron exist in the liquid state. The solidus below that iron-carbon exist in solid state is well-known as AEPGCH. Among these two lies there exist mixes of liquid and solid.
The line IK and GK are respectively designated as A3 and Acm. Austenite is unstable below the lines A3 and Acm if carbon content is less than 0.8 percent. Austenite begins to transform into ferrite on cooling and gets enriched in carbon along line A3 until point K is reached. Similarly for carbon content between 0.8 and 2.0% iron carbide will precipitate and carbon in austenite will vary until point K (carbon 0.8%) is reached. At point K austenite will transform into pearlite. Pearlilte is an intimate mixture of ferrite and cementite (Fe3C) having characteristics lamellar structures composed of alternate platelets of ferrite and cementite. The transformation reaction at K in which a single solid phase splits into two phases is termed eutectoid. The equation is written like:
Solid phase (A) → Solid phase (B) → Solid phase (C)
(Austenite) ( Ferrite) (Cementite )
Actually at any point K three phases exist (P = 3) and three are two constituents (C = 2), thus utilizing Gibb's phase rule the degree of freedom, F can be computed.
Gibb's rule at constant pressure,
P + F = C + 1
F = 2 - 3 + 1 = 0
Hence the eutectoid point like eutectoid point is non-variant.
a and g phases can dissolve carbon and the solubility changes along with temperatures. The solid solution of carbon in a-iron is known as ferrite whilst the solid solution of C in g-iron is called as austenite. Ferrite and austenite are also implemented as a and g respectively. The solubility of C in austenite is upto 0.2 percent while ferrite can dissolve C only upto 0.025 percent. The C solubility in austenite modifies beside GK in austenite and beside LN in ferrite. Beyond point Carbone on solidus, the compound Fe3C that is cementite, separates. Cementite is brittle and hard while ferrite on the extreme left of phase illustration is soft and ductile.
Austenite converts also into cementite beside the ling GK and into ferrite along IK.
Hence the eutectoid point like eutectoid point is non-variant.
Slightly beyond 110oC at point C on solidus, the eutectoid transformation happens. The liquid state at C contains 4.3 percent C and this liquid begins to convert into two solid phase. One phase is known as Ledeburite that is eutectic mixture of austenite and cementite whilst the other phase is cementite. On additionally cooling eutectic austenite transforms slowly in cementite, varying composition beside GK till it changes into pearlite and cementite on eutectoid point K.