Reaction rates
The rate of reaction usually depends on the concentration of reactants, frequently according to a power law like
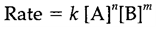
where k is the rate constant and n and m are the orders of reaction by respect to reactants A and B. Orders of reaction rely on the technique and are not essentially equal to the stoichiometric coefficients a and b. The rate constant depends on the method and particularly on the energy barrier or activation energy associated with the reaction pathway. The High activation energies (Ea) give low rate constants since only a small fraction of molecules have enough energy to react. This proportion may be increased by increment in the temperature and rate constants probably follow the Arrhenius equation:
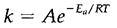
Large activation energies occur in reactions where covalent bonds must be broken before new ones are formed or in which atoms must move via solids. Reactions that are involving free radicals or ions in solution, frequently have small (sometimes zero) activation energies.
Reactions might be accelerated by the existence of a catalyst that acts by providing another pathway with lower activation energy. True catalyst by definition can be recovered unaffected after the reaction and so does not change the thermodynamics or the position of equilibrium.