Equilibrium constants
For a general reaction like
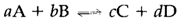
The equilibrium constant is
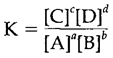
where the words [A], [B],...strictly stand for activities but are often approximated as concentrations or partial pressures. (this presumes ideal thermodynamic behaviour and is a much better approximation for gases than in solution.) Pure solids and liquids are not included in an equilibrium constant as they are present in their standard state.
A very large value (» 1) of K indicates a strong thermodynamic tendency to react, so that very little of the reactants (A and B) will remain at equilibrium. Conversely, a very small value (<<1) indicates very little tendency to react: in this case the reverse reaction (C and D going to A and B) will be very favorable. For any reaction K may be associated to the standard Gibbs free energy change (ΔGΘ) according to
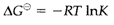
where R stand for gas constant (=8.314 J K-1 mol-1) and T is the absolute temperature (in K). So equilibrium constants can be estimated from tabulated values of 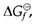 and trends may frequently be interpreted in terms of changes in ΔHΘ and ΔSΘ.
and trends may frequently be interpreted in terms of changes in ΔHΘ and ΔSΘ.
Equilibrium constants change by the temperature in a way that relies on ΔHΘ for the reaction. Within accordance with Le Chatelier's principle, K increases with increase in temperature for an endothermic reaction and decreases for the exothermic one.