Entropy and Free Energy
Entropy (S) is a measure of molecular 'disorder' or more accurately 'the number of microscopic arrangements of energy possible in a macroscopic sample'. Entropy increments with increase in temperature and rely strongly on the state.
Entropy changes (ΔS) are always positive for reactions that generate gas molecules. The Second Law of Thermodynamics states that the total entropy always increases in a spontaneous process and reaches a highest value at equilibrium. For apply this to chemical reactions it is necessary to include entropy changes in the surroundings caused by heat output or input. Both internal and external changes are taken account of by defining the Gibbs free energy change (ΔG): for a reaction taking place at constant temperature (T in kelvin)
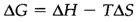
By the Second Law it can be displayed that ΔG is always negative for a possible reaction at constant pressure and temperature and without any external driving force like electrical energy and is zero at equilibrium.
As with enthalpies ΔS and ΔG for reactions do not rely on the reaction pathway taken and so can be estimated from thermodynamic cycles like that of diagram 1. They depend even more strongly than ΔH on pressure and concentration. Tabulated standard entropies might be employed to estimate changes in a reaction from

which is analogous to Equation 2 apart from that SΘ values are not zero for elements. The direct analogy to the Equation 2 may also be employed to calculate ΔGΘ for any reaction where the standard free energies of formation are known.