Enthalpy and Hess' Law
The enthalpy change (ΔH) in a reaction is equivalent to the heat input under circumstances of constant pressure and temperature. It is not precisely equivalent to the total energy change, as work may be completed by expansion against the external pressure. Corrections are usually small and enthalpy is generally employed as a measure of the energies involved in chemical reactions. Endothermic reactions (positive ΔH) are ones involving a heat input and exothermic reactions (negative ΔH) give a heat output.
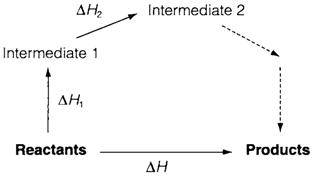
Hess' Law says that ΔH does not rely on the pathway taken between initial and final states and is a consequence of the First Law of Thermodynamics that asserts the conservation of total energy. Diagram 1 depicts a schematic thermodynamic cycle where the entire ΔH can be expressed as the sum of the values for individual steps:
It is significant that they need not present any feasible method for the reaction but can be any steps for which ΔH values are existing from experiment or theory. Hess' Law is often employed to estimate ΔH values that are not directly accessible, for instance, in connection with lattice energy and bond energy calculations.
Enthalpy alteration does depend on conditions of pressure, temperature, and concentration of the first and final states and it is significant to specify these. Standard states are described as pure substances at general pressure (1 bar) and the temperature must be additionally specified, even though 298 K is generally used. Improvements must be applied for any other conditions. The standard enthalpy of formation of any compound consider to formation from its elements all in standard states. Tabulated values permit the standard enthalpy change ΔHΘ in any reaction to be calculated from.

This follows from Hess' Law. By the definition, is zero for any element in its stable (standard) state.