Possible spin orientations:
Let us focus our attention on the methyl protons of ethyl chloride. In the absence of the neighbouring methylene group protons the methyl protons would come to resonance at a certain frequency depending on the effective field experienced by them. It will give a single signal in the low resolution spectrum. In the presence of methylene group with two equivalent protons the field experienced by the methyl protons gets altered because the nuclear spins of methylene protons also act as bar magnets. The magnitude of the effect would depend on the relative orientations of the nuclear spins of these protons. A nuclear spins of the two protons can have four possible combinations of spin orientations in the applied magnetic field as shown in Figure.
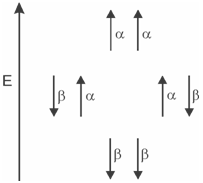
Figure: Possible spin orientations of two protons of the methylene group
In the first combination both the spins are in the α state. As these are aligned with the direction of the applied field, those would augment the magnetic field experienced through the methyl protons. As a result, the methyl protons would come to resonance at a lower field than that in the absence of methylene protons. Similarly, the fourth combination in which both the spins are in the β state would make the methyl group protons to come to resonance at a higher field. The other two combinations with one α and one β spins would have no net effect on the methyl protons absorption. Therefore, the resonance absorption signal of the methyl protons would be split up into three peaks (triplet) having relative areas in the ratio of 1:2:1. We are sure that you can justify the ratios of 1:2:1.