NMR Spectrum of dichloroethane:
Let us now refer the affect of the methyl protons on the methine proton. There are various probabilities here. The methyl protons could all be opposed to the applied ?eld or they could all be aligned along with the ?eld. Other possibility is for two of the protons to be with the ?eld when one is against the ?eld. Note that this kind of arrangement is three times more likely than comprising all the protons pointing similar way. At last, two of the protons could be against the ?eld and one could be with the ?eld. Again the possibility of this sort of arrangement is three times more likely than having all the nuclei pointing similar way. As there are four ways of orientating the three methyl protons, the signal for the methine proton is split into 4 different peaks (a quartet). These peaks will not be of equivalent intensity as there is more chance of specific orientations than others. The comparative intensities match the statistical probability of the different orientations that is 1:3:3: 1.
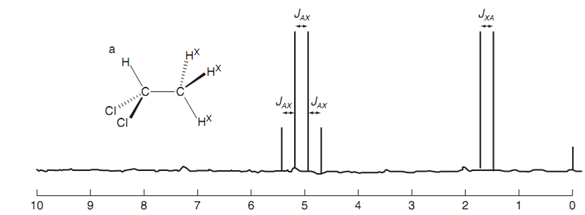
Figure: NMR Spectrum of 1,1-dichloroethane.
We can suppose the number of peaks in the methine signal (A) and their relative intensity in the following way shown in figure. If no coupling took place, the signal would be a singlet. Though, there are three neighboring protons because of the methyl group. We shall see what occurs while we split the methine signal with each of these methyl protons in turn. Coupling along with one of the methyl protons splits the signal in a doublet of intensity 1: 1. Splitting with a 2nd methyl proton splits each of these peaks into a doublet. Because the splitting is similar, the two inner peaks of each doublet overlap to provide a single peak such that we end up with a triplet comprising an intensity of 1:2: 1. Each of these peaks is now split through the effect of the third methyl proton to provide a quartet of ratio 1:3:3: 1. The peak at highest chemical shift corresponds to the arrangement in which the secondary magnetic ?eld of all three methyl protons is along with the applied ?eld and improving it. The peak at the next highest chemical shift corresponds to the situation in which two of the methyl protons are with the applied ?eld and third one is against it. The next peak has two protons against the applied ?eld and one proton with it. At last we have the peak at the lowest chemical shift that corresponds to the situation in which the magnetic ?elds of all three methyl protons oppose the applied ?eld. Note that the chemical shift for the signal is similar as it would have been if no splitting had occurred (that is the middle of the signal). The coupling constants JAX and JXA are similar for both signals as they are coupled together.