Instrumentation:
General procedure of NAA could be divided within various stages viz sampling, sample preparation, sample packing, irradiation and activity measurements, calculation of results, data processing and its interpretation. Of these irradiation and activity measurement are the two major steps of NAA which will be discussed here. Neutron irradiation could be carried out along with any of the neutron sources described in Table 13.1. Isotopic neutron sources could be used for some specific studies including analysis of major constituents in bauxite or pyrolusite or alloys. Most widely used sources are nuclear reactors. In India three nuclear reactors at the Bhabha Atomic Research Centre (BARC), Mumbai are APSARA, CIRUS and Dhruva which are used for NAA work. The γ-ray spectrometry forms the basis of NAA. However, a method based on β-activity measurement has been developed for the determination of P via 32P. In principle, a scintillation gamma ray spectrometer can be used but it has limited applicability when radiochemical separation is carried out. Generally an HPGe detector is used because of better performance in terms of energy resolution, peak to Compton ratio, efficiency and linear response of pulse height and energy of gamma photons. It consists of HPGe detector with all its electronic accessories as schematically shown in Figure. Radionuclides are identified by prior calibration of the spectrometer using standard sources. In some cases, however, half life of the radionuclide is also followed.
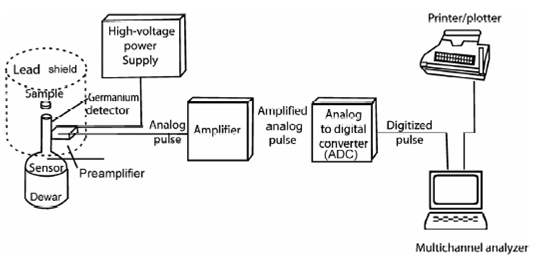
Figure: Schematic of counting set up for high resolution gamma spectrometry
Characteristic Eγ , t½ , and other characteristics of common elements determined by NAA are listed in Table 13.2. A photographic illustration of the HPGe detector surrounded with lead shielding, NIM Bin with HV supply, ADC and MCA including computer are shown in Figure.