Representation of Crevice Pitting:
In iron which is exposed to water, a same action could occur if adjacent areas of the metal surface become exposed to solutions along with different oxygen concentrations. For instance, the solution within a crevice exchanges slowly along with the bulk of the solution outside the crevice. Oxygen inside the solution inside a crevice will be depleted initially through the corrosion reaction.
2Fe + O2 → 2FeO
This reaction alone does not generate a protective film on the metal. Since of restricted flow within the crevice, replenishment of oxygen will be extremely slow; thus, the solution without the crevice will have a low oxygen concentration associative to which outside the crevice as displays in Figure. The two adjacent areas then built a concentration cell along with electrons flowing from the region of low oxygen concentration to the region of high concentration. Therefore, metal goes within solution (oxidation) within the crevice, and reduction occurs outside the crevice. Metal ions diffuse out of the crevice, more metal dissolves, and the procedure continues. This results within the formation of a pit in the crevice.
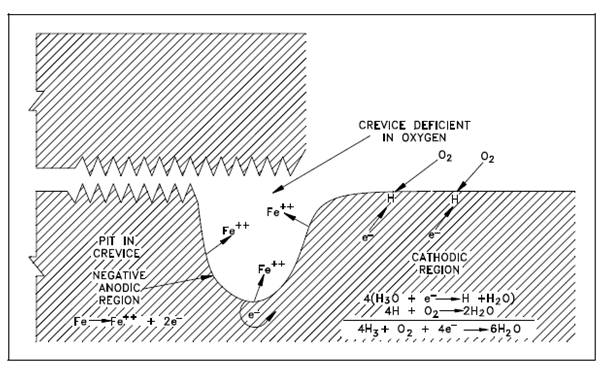
Figure: Representation of Crevice Pitting
The presence of oxygen could also promote pitting at areas on the metal surface which are initially anodic along with respect to an adjacent area. For instance, assume that adjacent areas on a metal surface exhibit slightly different oxidation potentials. Loss of metal, or Oxidation proceeds at the region of higher potential. Corrosion within the region of higher potential leads to establishment (at least initially) of a porous oxide film. A thickness of the film built on the adjacent cathodic region will be much less. Oxygen inside the bulk of solution could reach the cathodic surface (along with the thin film) more readily than it could the nearby anodic surface region (along with the thicker oxide film). A Depolarization of the cathodic region (thin film) through oxygen tends to manage this region cathodic, although a deficiency of oxygen below the thicker porous corrosion film assists in manage an anodic condition within this region. The whole output is corrosion or wasting away of the metal in the anodic region below the thicker film. Therefore, a pit within the metal surface is formed under the mound of surface oxide, as described in below figure. Pitting of this category is general in both low temperature and high temperature iron-water systems if precautions are not taken to erase the oxygen from the water inside the system.