Pitting and Crevice Corrosion:
The other probable effect of dissolved oxygen is accelerated localized attack. This is especially likely in the fields of limited circulation. A resulting corrosion is known as pitting corrosion.
Pitting corrosion occurs whereas the anodic site becomes fixed within a small area and the structure of holes (deep attack) in an or else unaffected area takes place. Crevice corrosion is a category of pitting corrosion which occurs specifically inside the low flow region of a crevice.
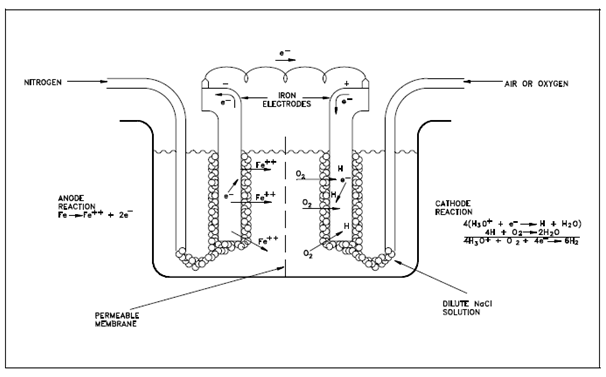
Figure: Differential Aeration Cell
To describe pitting attack, assume a special category of galvanic cell known as a differential aeration cell like as the one described in above figure. This particular differential aeration cell is displaying current flow as a output of depolarization of one electrode (cathode) through oxygen. In this category of cell, two iron electrodes are exposed to a dilute solution of an electrolyte (NaCl, for instance). Air (or oxygen) is bubbled around one electrode and nitrogen is bubbled around the other. A current flows by the wire linking the two electrodes. The difference within potential is an output of the difference in oxygen concentration at the two electrode surfaces. At the electrode exposed to nitrogen then electrons are given up through the iron as it is oxidized. Those electrons readily flow by the external circuit to the electrode exposed to oxygen. At this depolarized electrode they could participate in a reduction reaction. As an output, oxidation occurs at the electrode exposed to nitrogen and reduction occurs at the aerated electrode. The Oxidation at one electrode and reduction at the other creates a potential and a flow of current through the connecting wire. Remember that loss of metal occurs at the electrode which is deficient within oxygen.