Pit in Metal Surface:
It is also found in which certain ions, notably chloride ions that cause pitting of iron and steel. The exact mechanism through that this occurs is not clear, but in a few ways chloride ions cause defects within the passivating oxide layer on the metal surface area. The defects are highly localized and are surrounded through large passive areas which tend to be cathodic. Therefore, a small anodic (oxidation) site is surrounded through a large cathodic (reduction) area. A current density will then be extremely large at the anodic site, and attack on the metal will be rapid. In a few test cases, deep pits have been observed inside a few hours.
Pitting and crevice corrosion are a main hazard to a nuclear facility since of the rapid penetration of the metal along with little whole loss of mass. The nuclear facility minimizes pitting and crevice corrosion through the subsequent actions.
1 Preventing stagnant or low flow conditions.
2 Using metals and alloys which are less susceptible to the corrosion.
3 Avoiding agents in the medium which cause pitting (for instance, oxygen and chlorides).
4 Designing the system and elements such that no crevices are present.
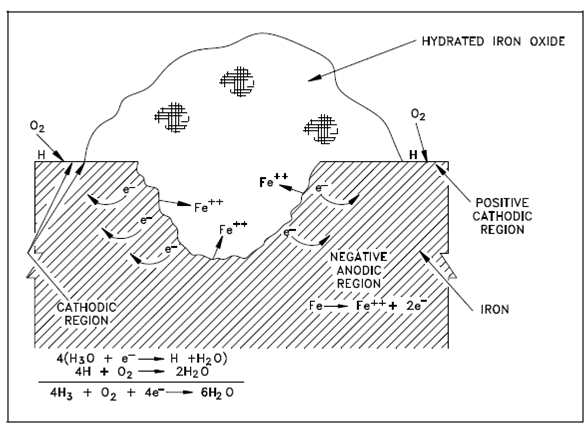
Figure: Pit in Metal Surface Promoted by Depolarization