Geometry:
In shape the 2p orbitals are dumbbell whereas the sp hybridized orbitals are deformed dumbbells along with one lobe much larger as compared to the other. The 2py and 2pz orbitals are at right angles to each other. The sp hybridized orbitals take the space that left over and are in the x axis point out in opposite directions (just only the major lobes of the sp orbitals are displayed in black).
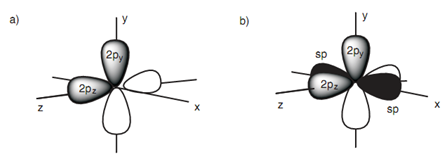
Figure: (a) 2py and 2pz orbitals of a sp hybridized carbon; (b) 2py, 2pz and sp hybridized orbitals of a sp hybridized carbon.
A molecule by using the 2 sp orbitals for bonding will be linear in shape. There are two general functional groups in which such bonding occurs - alkynes and nitriles.