Alkynes:
Let us refer the bonding in ethyne in which each carbon is sp hybridized. The C-H bonds are strong σ bonds in which each hydrogen atom employs its half-filled 1s orbital to bond along with a half-filled sp orbital on carbon.
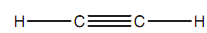
Figure: Ethyne.
The remaining sp orbital on every carbon is employed to form a strong σ carbon-carbon bond. The full σ bonding figure for ethyne is linear and can be simplified as displayed in the diagram.
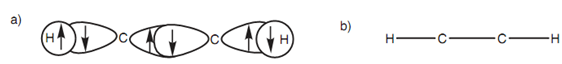
Figure: (a) σ bonding for ethyne; (b) representation of σ bonding.
Additional bonding is possible because each carbon has half-filled p orbitals. So, the 2py and 2pz orbitals of every carbon atom can be overlap side-on to form two π bonds. The π bond created through the overlap of the 2py orbitals is presented in dark gray. The π bond resultant from the overlap of the 2pz orbitals is presented in light gray. Alkynes are linear molecules and are reactive because of the comparatively weak π bonds.