Electrodialysis (ED):
In electrodialysis (ED) process, electrolytes are removed from one solution to the other and it is used for desalination of saline waters. The anions and cations permeate by cation selective and anion selective membranes, correspondingly as a result of applied electrical energy. Even by reverse osmosis and electrodialysis are both meaningful for desalination, there is a fundamental difference among the two. In RO, a solvent permeates by the membranes and solutes, both electrolytes and nonelectrlytes, are retained through the membranes. In ED, electrolytes permeate by the membranes and, the solvent and nonelectrolytes commonly do not permeate by the membranes. The solution from that electrolytes are erased gets depleted of salt and the solution that receives the solute gets enriched along with salt. A easy schematic of an electrodialysis procedure is given in Figure.
Concentrated feed solution
AE, CE= Anion selective and cation selective membranes; A,C = Anion and cation, respectively.
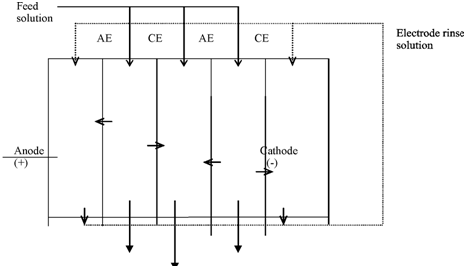
Figure: A schematic representation of electrodialytic process
The process is used for desalination as well as production of table salt. The cation and anion associative membranes used in this procedure are ion exchange membranes that are nothing but ion exchangers in sheet form. As same to cation exchange and anion exchange resins, those are cation exchange and anion exchange membranes.