Solvent efficiency and temperature:
Solvent efficiency is measured through α, the associative retention. It is the ratio of adjusted retention times or partition coefficients. Computation of solvent efficiency is illustrated in Figure.
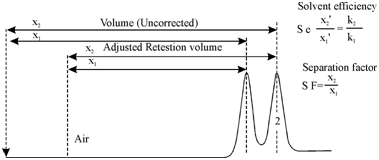
Figure: Calculation of solvent efficiency
Relative retention differs from the separation factor (S.F.), while S.F. is the ratio of uncorrected retention times. Here Both α and Kc are temperature dependent. Therefore, over a limited temperature range, α will be constant. The distribution constant, decreases along with increasing temperature, that is., the fraction of the solute in the gas •phase will increase and thus, the elution time will decrease. This results in decreased separation because it is the liquid phase that performs the separation. No separation occurs within the gas phase. To achieve better separations, lower temperatures should be used. Lower temperatures mean more liquid phase interaction, more separation and longer analysis time. At a minimum, the solute should spend 50 % of the time within the liquid phase so that the retention time exceeds double the retention time of air.