Thermodynamics
Refer an ionic solid that dissolves in water as per the equation:
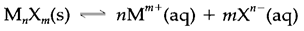
For this reaction the equilibrium constant,
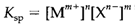
is known as the solubility result of MnXm. The form of this equilibrium is significant in understanding influences like the affect of pH and complexing (see below) and also the common ion effect: it can be observe that adding one of the ions Mm+ or Xn- will shift Reaction 1 to the left and so decrease the solubility of the salt. So AgCl(s) is much less soluble in a solution consisting of 1 M Ag+ (example from soluble AgNO3) than otherwise. Equilibrium constants within solution should accurately be written by using activities not concentrations. The variation among these quantities is large in concentrated ionic solutions and Ksp is quantitatively dependable as a guide to solubilities (calculated in concentration units) only for extremely dilute solutions. Yet, a thermodynamic analysis of the issues determining Ksp is helpful for understanding general solubility trends. As per to Ksp is associated to the standard Gibbs free energy change of solution. Diagram 1 displays a thermodynamic cycle which is relates the entire ΔG to two separate steps: (i) the formation of gas-phase ions; (ii) their consequent solvation. The enthalpy contributions include a balance among the lattice energy of the solid and the solvation enthalpies of the ions. In a solvent like water with a extremely high dielectric constant these contributions approximately cancel. Yet, some of the solubility trends shortened below can be understood in terms of the changing balance among lattice energies (proportional to 1/(r++r-), in which r+ and r- are radii of individual ions) and the sum of the individual solvation enthalpies (each approximately proportional to 1/r). For instance, a small ion has a large (negative) solvation energy, but while partnered by a large counterion cannot achieve an particularly large lattice energy. So, with ions of very dissimilar size, solvation is comparatively favored and solubility tends to be larger than with ions of identical size. Though, the entropy terms are, also significant. The first step in Fig. 1 includes an entropy increase,but solvation gives an ordering of solvent molecules and a negative ΔS contribution. Complete ΔS values for dissolving ions with multiple charges are generally negative, an influence that tends to lower the solubility.