Limiting ionic conductivities:
The limiting ionic conductivities can be obtained from the literature, the solubility c of a sparingly soluble salt can therefore be determined from conductivity values. Once c is known the solubility product can also be calculated as follows.
Consider the sparingly soluble salt solution of AgCl. The limited molar conductivities of its ions Ag+ and Cl-, are 0.00619 S m2 mol-1 and 0.00763 S m2 mol-1 respectively. The conductivity of AgCl solution at 298 K is measured to be 2.28 × 10-4 S m-1. To calculate solubility product, first calculate concentration of Ag+ and Cl- ions in the solution using Eq. ,
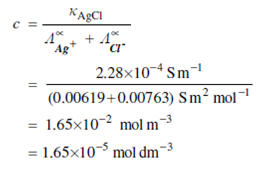
c = κ AgCl/ Λ Ag+∝ +Λ Cl-∝
=2.28×10-4 S m -1/(0.00619 + 0.00763) S m 2 mol -1
= 1.65×10-2 mol m -3
= 1.65×10 -5 mol dm -3