Racemization of an asymmetric center:
There are 2 steps in the SN1 mechanism that are shown in figure. The first step is the rate ascertaining step and includes loss of the halide ion. The C-I bond breaks along with both electrons in the bond moving onto the iodine atom to provide it a fourth lone pair of electrons and a negative charge. The alkyl portion turns into a planar carbocation in which all three alkyl groups are as far apart from each other as possible.
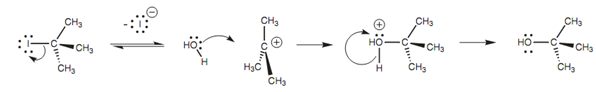
Figure: SN1 Mechanism.
Now the central carbon atom is sp2 hybridized with empty 2py orbital. In the 2nd step, water works as a nucleophile and reacts with the carbocation to make an alcohol.
In the mechanism that is displayed, we have shown the water molecule coming in from the left side, but because the carbocation is planar, the water can attack evenly well from the right hand side. Because the incoming nucleophile can attack from any side of the carbocation, there is no complete inversion of the carbon center. This is important when the reaction is performed on chiral molecules. For instance, if a chiral alkyl halide reacts with water through the SN1 mechanism, both enantiomeric alcohols would be made resultant in a racemate. Though, it has to be stated that total racemization does not generally occur in SN1 reactions. This is since the halide ion (departing from one side of the molecule) is still in the vicinity while the attacking nucleophile makes its approach. The result the departing halide ion can hinder the approach of the attacking nucleophile from that specific side. The word stereo specific points out that the mechanism results in one particular stereo chemical outcome (for example the SN2 mechanism). This is different from a reaction that is stereo selective in which the mechanism can lead to much more than one stereo chemical outcome, but where there is a preference for one outcome over other. Several SN1 reactions will show a slight stereoselectivity.
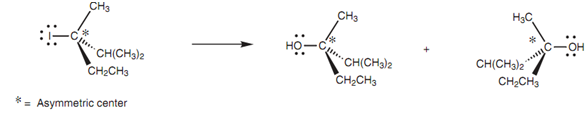
Figure: Racemization of an asymmetric center during SN1 nucleophilic substitution.