Fractionation by size exclusion chromatography:
The entire picture of fractionation by size exclusion chromatography can be visualized in some semiquantitative terms. So let VR be the retention volume for a solute along with a chromatographic column. Let V0 be the interstitial volume (void volume), that is, the volume within the column which is available to the mobile phase. VL is the volume of water within the gel particles available for accepting solutes. On the lines of GLC, we can write the following equation:
VR = V0 + KVL
where, K is some form of distribution coefficient. If the solute is completely excluded from the interior of gel then K = 0 and VR = V0. Such marker substances are available. Now, if the solute can freely enter the gel and there should be no preference for water inside or outside the gel and thus, K = 1, and VR = V0 + VL. Taking the case of molecules which can penetrate the gel to some extent but not freely, K values fall between 0 and 1.
In cases where sieving is the only phenomenon responsible for fractionation, K values greater than 1 would never be encountered. However, sometimes these values are obtained suggesting the occurrence of phenomena like adsorption, hydrogen bonding and ion exchange between the gel and the solute. Figure shows the typical behaviour of variation in the retention volume with the molecular weight of the solute.
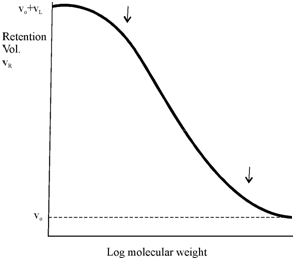
Figure: Relation between retention volume and molecular weight of the solute. The steep region between the arrows is the fractionation range.
If we refer to Figure, it is clear that the molecules of differing in size can be separated chromatographically in the sloping region of the curve. By varying the degree of cross linking of the polymer, the curve shifts horizontally. Here materials available which fractionate molecules in various molecular weight ranges. To Sephadex G. 50, the fractionation range for peptides and globular protein is molecular weight 1,500 to 30,000 although the range for G. 150 is 5,000 to 400,000.