Second messengers
The binding of ligands to several receptors leads to a short-lived raise in the concentration of certain intracellular signaling molecules known as second
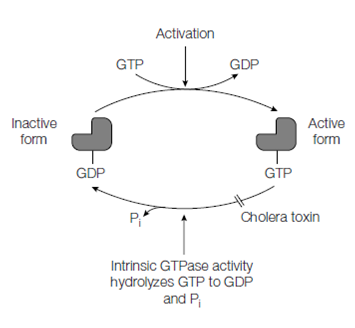
Figure: Cycling of the GTPase switch proteins between the active and inactive forms
messengers. The hormone or ligand can be considered as the best messenger. The major second messengers are 3',5'-cyclic GMP 2 (cGMP), 3',5'-cyclic AMP or cAMP, inositol 1,4,5-trisphosphate (IP3), 1,2-diacylglycerol (DAG) and Ca2+ . The elevation in the stages of one or other of these second messengers then leads to a rapid alteration in cellular function.
cGMP and cAMP are derived from ATP and GTP through the actions of adenylyl cyclase and guanylyl cyclase, respectively. For instance, the action of glucagon and epinephrine on glycogen metabolism is mediated by the second messenger cAMP that in turn activates the cAMP-dependent protein kinase, protein kinase A. cAMP and cGMP are short-lived as they are rapidly broken down through phosphodiesterases.
IP3 and DAG are derived from the membrane lipid phosphatidylinositol 4 or 5- bisphosphate which is a phosphorylated derivative of phosphatidylinositol through the action of phospholipase C that is also located in the plasma membrane and like adenylyl cyclase is activated through G proteins via GPCRs. One of the major actions of the polar IP3 is to diffuse by the cytosol and interact with Ca2+ channels in the membrane of the ER is causing the release of stored Ca2+ ions which in turn mediate various cellular responses. An DAG produced through the hydrolysis of phosphatidylinositol 4,5- bisphosphate, along with Ca2+ ions released from the ER make active protein kinase C a membrane-bound serine or threonine protein kinase which phosphorylates various target proteins over leading to alterations in a variety of cellular processes.
Calcium ions several extracellular signals induce a raise in the level of Ca2+ ions in the cytosol. For instance, in muscle cells, Ca2+switches contraction. The concentration of Ca2+in the cytosol is usually kept very low around. 0.1 μM, whereas it’s concentration in the extracellular fluid and in the lumen of the ER is high around. 1 μM. Thus there is a gradient of Ca2+ ions across the
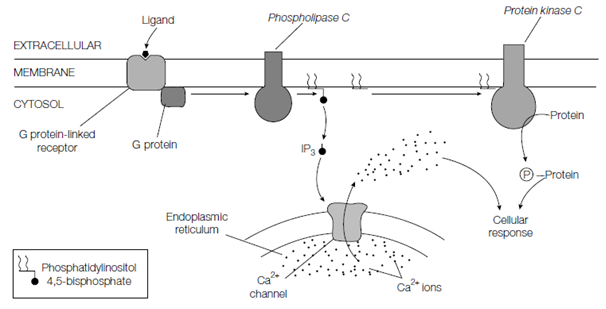
Figure: Generation of the intracellular second messengers inositol 1,4,5-trisphosphate (IP3), 1,2-diacylglycerol (DAG) and Ca2+ .
plasma membrane and ER membrane, like which when Ca2+ channels in these membranes are triggered to open, Ca2+ ions rapidly ?ow into the cytosol, raising the Ca2+ concentration through 10–20-fold and switching Ca2+-responsive proteins inside the cell. There are three main types of Ca2+ channel: voltage-dependent Ca2+ channels in the plasma membrane which open in response to membrane 2 epolarization, for instance in nerve cells; IP3-gated Ca2+ -channels in the ER and ryanodine receptors so called because they are inhibited by the plant alkaloid ryanodine that release Ca2+ from the sarcoplasmic reticulum in muscle cells or the ER of other cells. Ca2+ pumps, such as the Ca2+ -ATPase, in the ER and plasma membrane help to maintain the low concentration of Ca2+ ions in the cytosol of resting cells. Ca2+ - binding proteins serve as transducers of the cytosolic Ca2+ signal. These Ca2+ - binding proteins include troponin C in skeletal muscle (see Topic A3) and calmodulin a ubiquitous protein establish in all eukaryotic cells. The Calmodulin methods as a multipurpose intracellular Ca2+ receptor, mediating many Ca2+- regulated procedure and undergoes a conformational change upon binding Ca2+. The activated calmodulin can then bind to a number of different target proteins, including a family of Ca2+ or calmodulin-dependent protein kinases (CaM kinases) which then phosphorylate serines or threonines on other proteins.