Regulated proteolysis
There are various unusual signaling pathways for relaying signals from cell surface receptors to the interior of the cell which include regulated proteolysis. Between these pathways is which mediated through the receptor protein Notch and the pathway activated through secreted Hedgehog proteins which have been highly conserved across evolution and play crucial roles in animal development. In case of the transmembrane Notch receptor the binding of its ligand Delta to the extracellular face leads to a proteolytic cleavage first in the region adjacent to the membrane and then a second cleavage within the hydrophobic transmembrane region. The released cytoplasmic domain then migrates to the nucleus where it activates the transcription of several goals genes shown in figure.
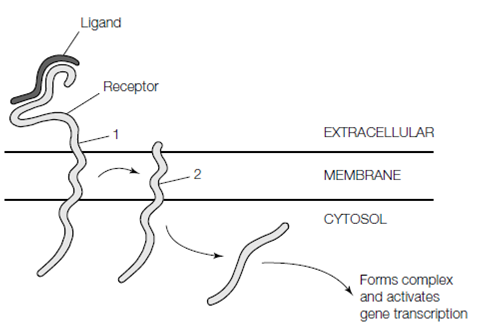
Figure: Cell signaling through the Notch receptor. Binding of ligand results in proteolytic cleavage of the receptor (1) on the extracellular face of the membrane. The resulting membrane-bound stub is then cleaved within the transmembrane domain (2), releasing the cytosolic tail which forms a complex with other proteins and activates gene transcription in the nucleus.
The Proteolytic cleavage within hydrophobic transmembrane region is unusual but more and more proteins are being identified which are subject to this regulated intramembrane proteolysis (RIP). The receptor cannot be reused as peptide bonds in the receptor protein are cleaved in this procedure.