Enzyme-linked receptors
Numerous receptors have intrinsic or tightly related enzyme activity. On the binding of ligand to their extracellular face such receptors undergo a conformational modify and activate an enzyme activity. In case of the insulin receptor that is a complex of two α- and two β-subunits held together through disulfide bonds the polypeptide hormone insulin the ligand binds to the extracellular face of the α-subunits. The receptor then undergoes a conformational modify leading to the autophosphorylation of the
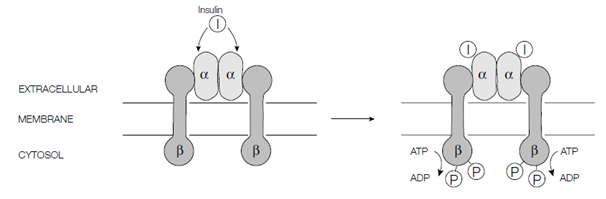
Figure: Signal transduction through an enzyme-linked receptor such as the insulin receptor.
cytosolic domain of the β-subunit. By the Specifically hydroxyl groups in the side chains of certain tyrosine residues are phosphorylated with ATP being the phosphate donor. Phosphorylated receptor is then identify through other proteins in the cytosol which in turn modulate several intracellular events permitting the cell to respond to the hormone appropriately. Additionally, β-subunit can straight phosphorylate other goal proteins within the cell.
The insulin receptor is an instance of a receptor tyrosine kinase, although the transforming growth factor TGF-β family of receptors have serine or threonine kinase activity in their cytosolic domain. Another receptors with strongly related enzyme activity involved several cytokine receptors which bind interferons and growth hormone some interleukins and other cytokines. For several ligand binding, enzyme- linked receptors, induces the oligomerization the formation of dimers or higher oligomers and it is this rearrangement of the cytosolic domains which enables the neighboring kinase domains of the receptor chains to cross-phosphorylate every other in the procedure of autophosphorylation.
Some enzyme-linked receptors as well as other receptors interact with scaffold proteins inside the cell that organize groups of proteins into signaling complexes. The proteins within these signaling complexes assemble by the interactions of a variety of highly conserved and small binding domains like as the (Src homology 2) SH2 domains and PTB phosphotyrosine-binding domains which bind to phosphorylated tyrosine residues, SH3 (Src homology 3) domains which bind to short proline-rich amino acid sequences and pleckstrin homology (PH) domains which bind to the headgroups of inositol phospho- lipids which have been additional phosphorylated through phosphatidylinositol 3-kinase PI 3-kinase. Many scaffold proteins contain multiple PDZ domains, each of that binds to a specific motif on a receptor or signaling protein. By the binding of these scaffold proteins to the activated receptor may help to relay the signal onward or may decrease the signaling procedures giving negative feed- back.
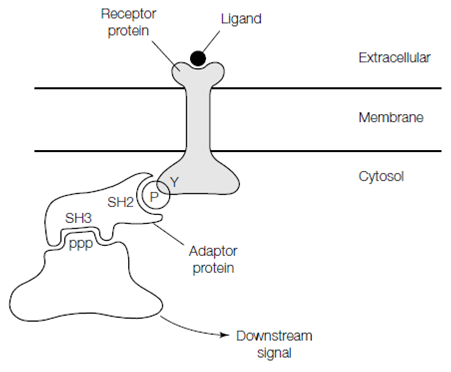
Figure: A hypothetical signaling complex. Binding of ligand to the extracellular face of the cell surface receptor results in phosphorylation of a tyrosine residue in its cytosolic domain. The phosphorylated tyrosine residue is recognized by an SH2 domain in the adaptor protein. Elsewhere in the adaptor protein is an SH3 domain that binds to a proline-rich sequence (PPP) in another signaling protein, such that the signal is relayed into the cell.
Some cell surface receptors needs tyrosine phosphorylation for their activity and still lack a tyrosine kinase domain. That receptors act by cytoplasmic tyrosine kinases or nonreceptor tyrosine kinases that relates with the receptor and phosphorylate several goal proteins. The biggest family of cytoplasmic tyrosine kinases is the Src family which involves Src, Yes, Fyn and Lck, that all contain SH3 and SH2 domains and are situated on the cytoplasmic surface of the plasma membrane.