Semiconductors:
Semiconductors are substances whose conductivity lies among insulators (very poor conductor of electricity) and metals (very good conductor of electricity). Some instance are Silicon (Si), Germanium (Ge) and Gallium Arsenide (GaAs).
Let us try to see what is the difference among semiconductors, insulators and conductors in terms of energy band diagrams. In an atom, electrons may have only certain discrete values of energy called the energy states or energy levels. While atoms form a substance these energy levels split into several close allowable energy bands. These bands are also separated by forbidden energy gaps called forbidden band (FB). Note down that electron energies are expressed in terms of electron volt (eV) and
1 eV = 1.602 × 10- 19 C × 1 V = 1.602 × 10 -19 Joules
Electrons occupy the lowest energy levels first. The lower energy bands with the most filled energy states are called as the valence band (VB). The higher energy state with the most empty energy states is called as conduction bands (CB). The difference in energy among the VB and CB is called the energy gap (Eg). An electron in the VB needs energy equal to or higher than Eg to jump to the CB. While an electron from VB moves to the CB, a hole is made in the VB because of the absence of electron. Holes are positive charge carriers in Semiconductor while electrons are the negative charge carriers. Typical semiconductor has an Eg in 0.1 eV - 3.5 eV, for insulator, Eg > 5 - 6 eV and for metals or conductors there is an overlap between the CB and VB as depicted in Figure.
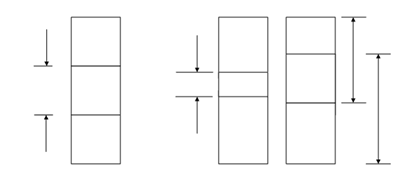
(a) (b) (c)
Figure: Energy Band Diagram of (a) Insulator; (b) Semiconductor; and (c) Metal or Conductor