Entropy
One effect of the second law is the expansion of the physical property of matter termed entropy (S). The entropy was established to help and describe the Second Law of Thermodynamics. The change in this property is employed to establish the direction in which a given procedure will continue. Entropy can also be described as a measure of the unavailability of heat to execute work in a cycle. These shares to the second law as the second law forecasts which not all heat given to a cycle can be converted into an equivalent quantity of work, some heat refusal should occur. Alter in entropy is stated as the ratio of heat transferred throughout a reversible process to the absolute temperature of the system.

Here:
ΔS = the change in entropy of a system during some process (Btu/°R)
ΔQ = the amount of heat added to the system during the process (Btu)
Tabs = the absolute temperature at which the heat was transferred (°R)
The second law can also be stated as ? S≥ O for a closed cycle. In another word, entropy should rise or remain similar for a cyclic system; it can never reduce.
Entropy is a property of a system. This is an extensive property which, similar to the total internal energy or total enthalpy, might be computed from specific entropies depend on a unit mass amount of the system, and hence S = ms. For pure substances, values of the specific entropy might be tabulated all along with specific enthalpy, specific volume, and other thermodynamic property of interest. One position to find this tabulated detail is in the steam tables. The figure is as shown below.
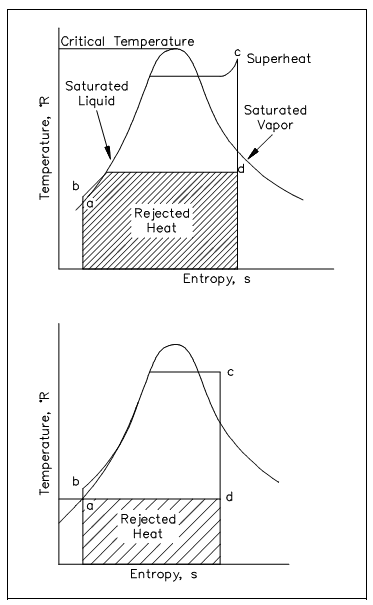
Specific entropy, since it is a property, is favorably used as one of the coordinates whenever symbolizing a reversible procedure graphically. The region under a reversible process curve on the T-s diagram symbolizes the amount of heat transferred throughout the process.
Thermodynamic problems, procedures, and cycles are frequently investigated by replacement of reversible procedures for the real irreversible process to help the student in a second law analysis. This replacement is particularly helpful since only reversible processes can be represented on the diagrams (h-s & T-s, for illustration) employed for the analysis. Real or irreversible processes can't be drawn as they are not a sequence of symmetry conditions. Only the first and final conditions of irreversible procedures are known; though, some thermodynamics texts symbolize an irreversible procedure by dotted lines on the diagrams.