Diagrams of Ideal and Real Processes
Any ideal thermodynamic process can be taken as a path on a property diagram, like a T-s or an h-s diagram. The real process which approximates the ideal process can also be symbolized on the similar diagrams (generally with the use of dashed lines).
In an ideal process including either a reversible expansion or a reversible compression, the entropy will be stable. Such isentropic processes will be symbolized by vertical lines on either T-s or h-s diagrams, as entropy is on the horizontal axis and its value do not alter. The real expansion or compression process operating among the similar pressures as the ideal process will appear much similar, though the dashed lines symbolizing the real process will slant a little towards the right as the entropy will rise from the beginning to the end of the process. The figures shown below illustrate ideal and real expansion and compression procedures on T-s and h-s diagrams.
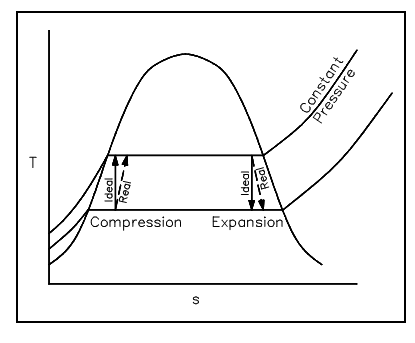
Figure: Expansion and Compression procedures on T-s Diagram
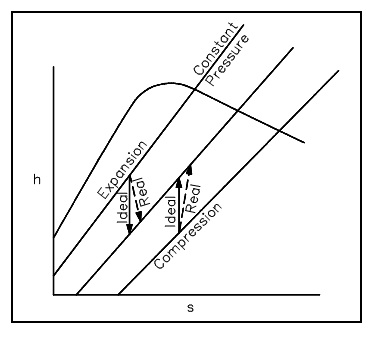
Figure: Expansion and Compression procedures on h-s Diagram