Hydride generation technique:
This gives a method for introducing samples containing As, Sb, Sn, Se, Bi and Pb within an atomiser as their representative hydrides. This enhances detection limits through a factor of 10 to 100. As some of these elements are highly toxic and occur in the environment at extremely low levels, their determination at low concentrations is extremely important. The volatile hydrides of these components may be generated by the reaction of an acidified aqueous solution of the sample to 1% aqueous solution of NaBH4 in a glass vessel. A representative reaction of As (III) with NaBH4 to form arsine (AsH3) is as follows.
3NaBH4+3HCl+4H3AsO3 → 3H3BO3+4AsH3+3H2O+3NaCl
The volatile hydrides like as AsH3, BiH3, H2Se, SbH3 etc. are swept out of the solution into the atomisation chamber through an inert gas carrier. The chamber is usually a silica tube heated within a tube furnace or in a flame where hydride gets decomposed leading to the creation of analyte element whose concentration is then determined from the atomic absorption signal. The signal is a peak same to that obtained with electrothermal atomisation. Schematic figure of primary system used for the hydride generation and atomisation is shown in Figure.
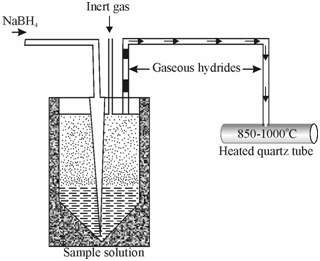
Figure: Schematic diagram of a basic system for hydride generation technique
Commercial hydride generation system use either electrically heated or flame heated quartz tube for atomisation. Its major benefit is enhanced sensitivity and freedom from matrix interferences as the element is separated from all other accompanying elements. In Table are given the comparison of detection limits by hydride generation and graphitic furnace AAS.