Wade's rules
The actually bewildering variety of structures adopted by boron-hydrogen compounds (boranes) can be rationalized by recognizing some main families, demonstrated by the series in Figure. 2.
- Closo boranes with n boron atoms adopt closed polyhedral structures based on triangular faces like the trigonal bipyramid (five vertices), octahedron (six) and icosahedron (12); such type of polyhedra are called deltahedra. The simple most instances are the ions [BnHn]2- like [B6H6]2- illustrated.
- In nido ('nest-like') boranes n boron atoms are found approximately at the positions of the vertices of an n+1-vertex deltahedron, with one vertex missing. The simple most general formula type is BnHn+4; for instance, B5H9, in which the boron atoms are placed at five of the corners of an octahedron.
- Arachno ('web-like') boranes are still more open and can be imagined like deltahedra with two vertices missing. They create a general series of formula BnHn+6 (e.g. B4H10).
Wade's rules give an electronic rationalization of the regularities that is based on the MO prediction that an n atom deltahedron by s and p valence orbitals, should have n+1 skeletal bonding MOs. For instance, in [B6H6]2- there are seven such type of MOs, and electrons might be counted as follows: there are the 26 valence electrons; 12 are assigned to 'normal' two-center B-H bonds, leaving 14 for skeletal bonding. There is no easy way of assigning these 14 electrons to localized two-center or even three-center bonds. Generally, we see that closo boranes with n atoms should have 2n+2 skeletal bonding electrons. Isoelectronic substitutions of atoms should maintain the structure; for instance, B10C2H12 is based on similar icosahedron as [B12H12]2-.
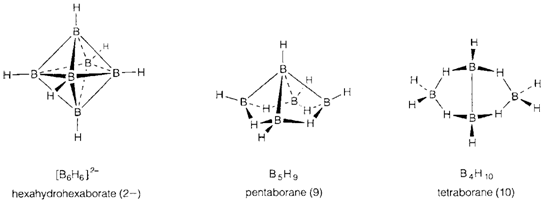
As Starting by the closo ion [BnHn]2- we can imagine removing one [BH]2+ unit and adding 4 H+ to give the n-1 nido borane Bn-1Hn+3 (example [B6H6]2- gives B5H9). Neither of these operations should change the number of skeletal bonding electrons, so B5H9 has 14, similar number like [B6H6]2-, and generally nido boranes with n atoms should have 2n +4 skeletal bonding electrons. The argument might be repeated, starting from nido BnHn+4, removing BH2+ and adding 2 H+, leading to the additional conclusion that arachno boranes with n atoms should have 2n+6 skeletal bonding electrons.
Wade's rules might be applied to 'naked' clusters created by p-block elements if it is assumed that each atom has one localized nonbonding electron pair. Thus in [Pb5]2- there are 22 valence electrons, 10 employed in lone-pairs, hence 12 for skeletal bonding: a closo structure is supposed, as found (4). In [Sn9]4- an identical count gives 22 skeletal bonding electrons, subsequent to 2n+4 and therefore the nido structure observed (7). It should be noticed that there are exceptions. The Extension to transition metal clusters requires accommodating the d bonding electrons and leads to the Wade-Mingos rules.