Aromatic rings
The Hückel MO approach treats the π electrons of rings like benzene. We imagine a framework of σ bonds created by sp2 hybrids on each carbon atom. The six remaining 2p π orbitals overlap to create six delocalized MOs. Diagram 1a depicts the pattern of orbital energies predicted. The lowest energy MO is created by combining all 2p orbitals with positive overlap to give full bonding; higher energy MOs are increasingly less bonding and more antibonding. Diagram 1 depicts the assignment of six π electrons like in the ground state of benzene. Aromatic stability arises since the electrons are collectively more stable in these MOs than they would be within the three separate double bonds.
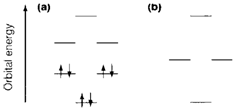
Fig. 1. Energies of π MOs in (a) benzene, (b) a four-membered ring compound.
For benzene the arrangement of MO energies is paralleled with other ring sizes: in every case there is a single orbital of lowest energy followed by pairs of equivalent energy. Figure 1b depicts the energies for a four-membered ring. four π electrons' assignment does not lead to a closed-shell ground state where every MO is either empty or filled, and certainly the four-π-electron molecule cyclobutadiene C4H4 is extremely unstable. This kind of argument leads to the Hückel 4n+2 rule: irrespective of the ring size, aromatic stability needs 4n+2 π electrons, in which n is a whole number. Feasible values are the 2, 6, 10,...but not 4, 8,.... One consequence is that the cyclopentadienyl fragment C5H5 is stable like a 6-π- electron anion [C5H5]-, an significant ligand for organometallic compounds.
There are instances of inorganic rings that conform to the Hückel rule. Heteroatom molecule B3N3H6 is isoelectronic with benzene even though much more reactive due to the polarity in the B-N bonds. The S2N2 ring (2) is an instance of a six-π-electron system even though the ring is four-membered. Electrons can be counted by the assigning two each to four localized S-N σ bonds and two electrons to a lone-pair on every atom. From the 22 valence electrons, six remain for delocalized π system. Ions [S4]2+ and [Se4]2+ have similar valence electron count as S2N2 and are also square planar.