Resonance interactions for the phenolate ion:
Phenol is less acidic as compared to the ethanoic acid however is more acidic than ethanol. Again, resonance can describe these variations. The conjugate base of phenol is termed as the phenolate ion. In this case, the resonance process can be performed several times to place the negative charge on 4 separate atoms - one oxygen atom and three of the aromatic carbon atoms shown in figure. The reality that the negative charge can be spread over four atoms might suggest that the phenolate anion have to be more stable as compared to the carboxylate anion, because the charge is spread over more atoms. Though, with the phenolate ion, three of the resonance structures place the charge on a carbon atom that is much less electronegative as compared to an oxygen atom. These resonance structures will be far less significant than the resonance structure comprising the charge on oxygen. The result of it is, delocalization is weaker for the phenolate ion than it is for the ethanoate ion. Yet, a fixed amount of delocalization still occurs which is why a phenolate ion is more stable as compared to an ethoxide ion.
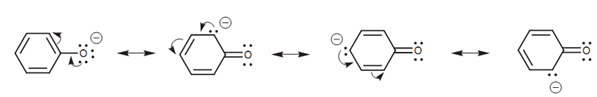
Figure: Resonance interactions for the phenolate ion.