Amines and amides:
Amines and amides are extremely weak acids and just only react with very strong bases. The pKa values for ethanamide and ethylamine are 15 and 40, correspondingly that means that ethanamide has the more acidic proton. This can be described via resonance and inductive effects.
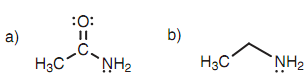
Figure: (a) Ethanamide; (b) ethylamine.
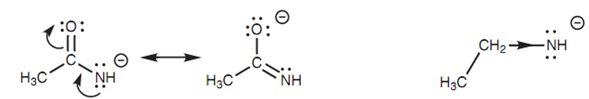
Figure: (a) Resonance stabilization for the conjugate base of ethanamide; (b) inductive destabilization for the conjugate base of ethylamine.