Type of functional group:
Beyond the primary question of whether the resin is a cation or anion exchanger, the effect of functional groups upon the selectivity of the resin is largely a matter of acid and base strength. A difference among the weak and strong exchange resin is rather sharp. Other than there are shades of strength in both the categories. These differences are largely reflected in the position of hydrogen or hydroxyl ion occupies in the series. For describes this point, a typical example can be cited. In Dowex-1 (a strong anion exchanger) all the three groups are methyl groups.
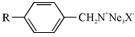
Dowex-2 differs only in that one of the methyl groups is replaced by an ethanol group. This substitution of methyl group changes the selectivity to give a resin which can be converted to the free base form much more efficiently.