Synthesis of tribromobenzene:
This problem can be conquers through using a strong activating group that will direct ortho/para and that can then be removed at the end of the synthesis. The amino group is perfect for this and the full synthesis is displayed in figure.
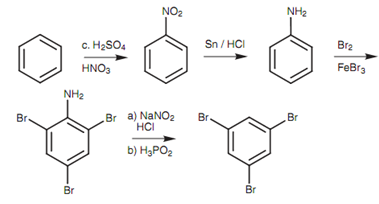
Figure: Synthesis of 1,3,5-tribromobenzene.
The synthesis of ortho-bromotoluene demonstrates how a sulfonic acid can be employed in a synthesis. O-Bromotoluene could possibly be synthesized through bromination of toluene or through Friedel-Crafts alkylation of bromobenzene.
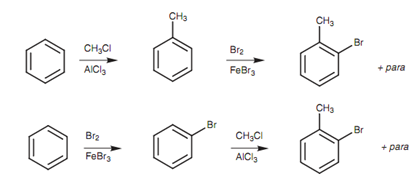
Figure: Possible synthetic routes to ortho-bromotoluene.
Though, the reaction would as well give the para-substitution product and this is more similarly if the electrophile is hindered from approaching the ortho position through unfavorable steric interactions. Other strategy would be to deliberately substitute a group at the para position of toluene before performing the bromination. This group would then work as a blocking group at the para position and would compel the bromination to occur ortho to the methyl group. If the blocking group could then be eliminated, the needed product would be acquired. The sulfonic acid group is specifically helpful in this respect because it can be easily removed at the end of the synthesis.