Synthesis of ortho-bromotoluene:
The sulfonation of toluene could in theory occur at the ortho position also the para position. Though, the SO3 electrophile is bulky and thus the latter position is preferred for steric reasons. One time the sulfonic acid group is present, both it and the methyl group direct bromination to similar position (ortho to the methyl group = Meta to the sulfonic acid group).
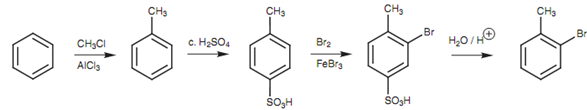
Figure: Synthesis of ortho-bromotoluene