Transcription factors have multiple domains:
In most cases, the transcription factors in eukaryotes which bind to promoter or enhancer sequences are activator proteins which induce transcription. That proteins commonly have at least two distinct domains of protein structure, a DNA- binding domain which recognizes the specific DNA sequence to bind to and an activation domain responsible for bringing about the transcriptional activation through interaction with other transcription factors and the RNA polymerase molecule. Several transcription factors operate as dimers, either homodimers (identical subunits) or heterodimers (dissimilar subunits) with the subunits held together through dimerization domains. The DNA dimerization domains and binding domains contain characteristic protein structures (motifs) which are described
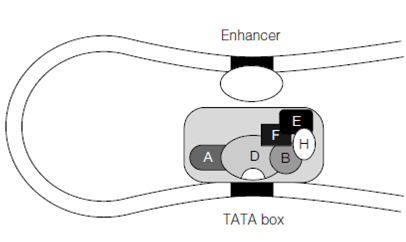
Figure: Looping out of DNA allowing the interaction of enhancer-bound factor(s) with the transcription initiation complex.
below. At last, some transcription factors example for steroid hormone receptors are responsive to specific short molecules (ligands) that regulate the activity of the transcription factor. In these cases, the ligand binds at a ligand-binding domain.