Dimerization domains:
Leucine zippers
The leucine zipper motif contains a leucine every seventh amino acid in the main sequence and forms an -helix with the leucines presented on the similar side of the helix every second turn providing a hydrophobic surface. Transcription factor dimer is created through the two monomers interacting via the hydrophobic faces of their leucine zipper motifs in the figure. In case of bZIP proteins, every monomer also has a basic DNA binding domain located N- terminal to the leucine zipper. Therefore the bZIP protein dimer has two basic domains. These really face in opposite directions that permits them to bind to
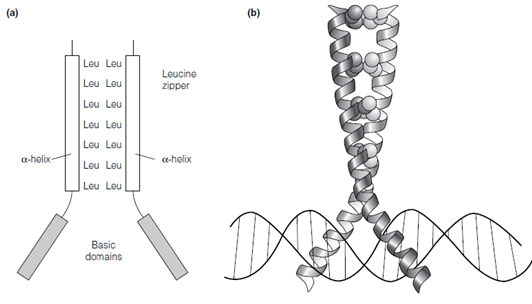
Figure: (a) A bZIP protein dimer showing the leucine zipper dimerization domain and the two basic domains; (b) folded structure of a bZIP protein showing the basic domains binding in the major groove of the target DNA. Reprinted from A. Travers, DNA-Protein Interactions, Chapman & Hall, 1993 with permission from A. Travers
DNA sequences which have inverted symmetry. They bind in the main groove of the goal DNA that shown in the above figure. The leucine zipper domain also acts as the dimerization domain in transcription factors which use DNA binding domains other than the basic domain. For instance: some homeodomain proteins that containing the helix- turn-helix motif for DNA binding have leucine zipper dimerization domains. In the case the dimers which form should be heterodimers or homodimers.