Aspartate transcarbamoylase
Aspartate transcarbamoylase ATCase aspartate carbamoyltransferase, a key enzyme in pyrimidine biosynthesis gives a good instance of allosteric regulation. The ATCase catalyzes the formation of N-carbamoylaspartate from aspartate and carbamoyl phosphate and is the committed step in pyrimidines biosynthesis in the figure. Binding of the two substrates carbamoyl and aspartate phosphate is cooperative, as described through the sigmoidal curve of V0 against substrate concentration. ATCase consists of six catalytic subunits and six regulatory subunits. The enzyme is feedback-inhibited through the end-product of the pathway, CTP which is also known as cytosine triphosphate that acts as an allosteric inhibitor. This molecule connects to the regulatory subunits and causes a decrease in the catalytic activity of ATCase through decreasing the affinity of the catalytic subunits for substrate molecules. In compare, ATP, one of the intermediates earlier on in the pathway which acts as an allosteric activator enhancing the affinity of ATCase for its substrates and leading to an increase in activity describe in the figure. ATP competes with the similar binding site on the regulatory subunit as CTP. High stages of ATP signal to the cell in which energy is available for DNA replication and so ATCase is activated resulting in the synthesis of the needed pyrimidine nucleotides. Whenever pyrimidines are abundant the high levels of CTP inhibit ATCase avoiding needless synthesis of N-carbamoylaspartate and subsequent intermediates in the pathway
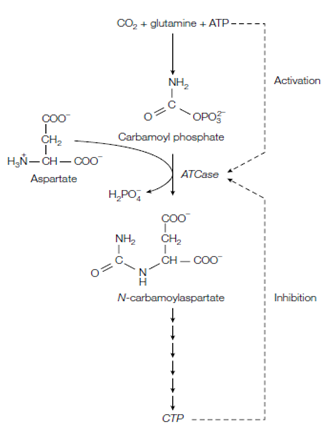
Figure: Formation of N-carbamoylaspartate by aspartate transcarbamoylase (ATCase) is the committed step in pyrimidine biosynthesis and a key control point.