Example of Standard Addition Method:
Within the determination of sodium in an unknown solution, the subsequent series of solutions were prepared. Every solution holds 10 cm3 of the unknown solution to that increasing volumes of 25 ppm sodium (as sodium chloride) salt solution is added and the total volume is made up to 25 cm3. An emission reading of all these solutions is provided below. Determine the concentration of sodium within the unknown solution.
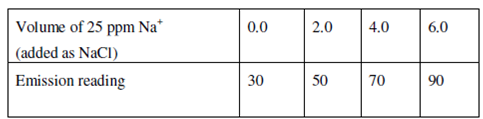
Solution
The curve of emission reading versus the concentration of added sodium ion (in ppm) is given in Figure.
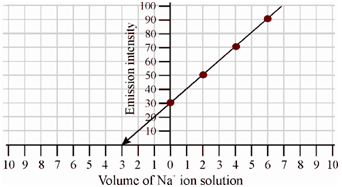
Figure: Schematic calibration plot of Emission vs. Concentration in the determination of sodium by standard addition method
On extrapolating the curve to zero emission intensity, that meets the x-axis on the negative side of the concentration axis. A concentration of sodium is found to be 3.0 ppm. Because the given (unknown) solution is diluted from 10 ml to 25 ml within the final solution, the concentration of sodium is 3 × 25/10 = 7.5 ppm.