Compression System:
The evaporator and the condenser are heat exchangers that evaporate and condense the refrigerant while absorbing and rejecting the heat. The compressor takes the refrigerant from the evaporator and raises the pressure adequately for the vapour to condense in the condenser. The expansion apparatus controls the flow of condensed refrigerant at this higher pressure back into the evaporator. Some typical expansion devices are throttle valves, capillary tubes and thermostatic expansion valves in case of large refrigeration systems.
Given fig (b) shows the T-S plot of the functioning of such a system. Here, the dry saturated functioning medium at state 1 is compressed isentropically to state two. Constant pressure heat transfer take place from state two till the compressed vapour becomes saturated liquid or condensate at state four. The compressed vapour is next throttled through the high pressure region within the condenser (state 4) to the low pressure region within the evaporator (state 5). As throttling is an irreversible procedure, this is represented by a broken line. Later than throttling to evaporator pressure, the heat transfer in the evaporator is responsible to vaporization of the working medium till state one is attained, therefore completing the cycle.
Referring to figure (b), considering ideal processes, we might see that:
q1-2 = 0 , being an isentropic procedure and,
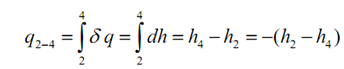
In the above equation the negative sign represents heat transfer from the system to the surrounds.
The procedure 4-5 is supposed to be adiabatic throughout throttling, an isenthalpic procedure.
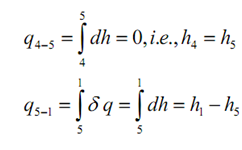
The heat transfer throughout 5-1 is the needed refrigeration effect. Again, by using first law of thermodynamics, we obtain the following:
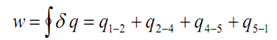
= 0 - (h2 - h4) + (h1 -h5) = - (h2 - h1)
here the -ve sign show that work is done on the system to execute the cyclic procedure. Then,
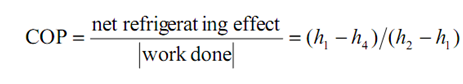
In the above described equation, the quantities h1 & h4 are known for respective pressures p1 (evaporator pressure) & p2 (condenser pressure). The state h2 is set up from the intersection of constant entropy line flowing through state 1 and pressure line p2.