Reduction of an amide to an amine:
The mechanism shown in figure involves addition of the hydride ion to make an intermediate that is transformed to an organoaluminum intermediate. The variation in this mechanism is the intervention of the nitrogen's lone pair of electrons. These are delivered into the electrophilic center to eliminate the oxygen that is then followed by the second hydride addition.
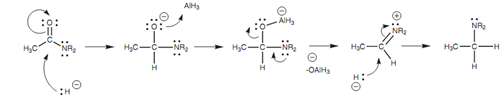
Figure: Mechanism for the LiAlH4 reduction of an amide to an amine.
Even though acid chlorides and acid anhydrides are transformed to tertiary alcohols with LiAlH4, there is slight synthetic benefit in this since similar reaction can be acquired on the more readily available esters and carboxylic acids. Though, since acid chlorides are much more reactive as compared to carboxylic acids, they can be treated along with a milder hydride-reducing agent and this permits the synthesis of aldehydes. The hydride reagent employed (lithium tri-tert-butoxyaluminum hydride) consists of three bulky alkoxy groups that lowers the reactivity of the remaining hydride ion. The meaning of this is the reaction stops after nucleophilic substitution with one hydride ion. Another sterically hindered hydride reagent - diisobutylaluminum hydride (DIBAH) - is employed to reduce esters to aldehydes. Generally low temperatures are required to avoid over-reduction.