Reduction of an acid chloride to an aldehyde:
Borane (B2H6) can be employed as a reducing agent to transform carboxylic acids to primary alcohols. One benefit of using borane rather than LiAlH4 is the fact that the former does not decrease any nitro groups that might be present. LiAlH4 decreases a nitro group (NO2) to an amino group (NH2).
It is value noting that carboxylic acids and acid derivatives are not reduced through the milder reducing agent - sodium borohydride (NaBH4). Hence this reagent can be employed to reduce aldehydes and ketones without influencing any carboxylic acids or acid derivatives which might be present.
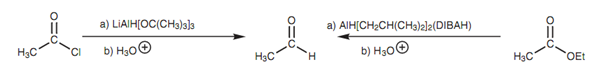
Figure: Reduction of an acid chloride and an ester to an aldehyde.