Oxidation:
Ketones are resistant to oxidation while aldehydes are simply oxidized. Treatment of an aldehyde along with an oxidizing agent causes in the formation of a carboxylic acid. Some compounds might be sensitive to the acid conditions employed in this reaction and an alternative way of performing the oxidation is to make use of a basic solution of silver oxide.
Both reactions include the nucleophilic addition of water to form a 1, 1-diol or hydrate that is then oxidized in identical way like an alcohol.
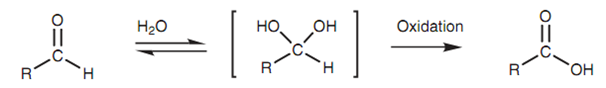
Figure: 1, 1-Diol intermediate.